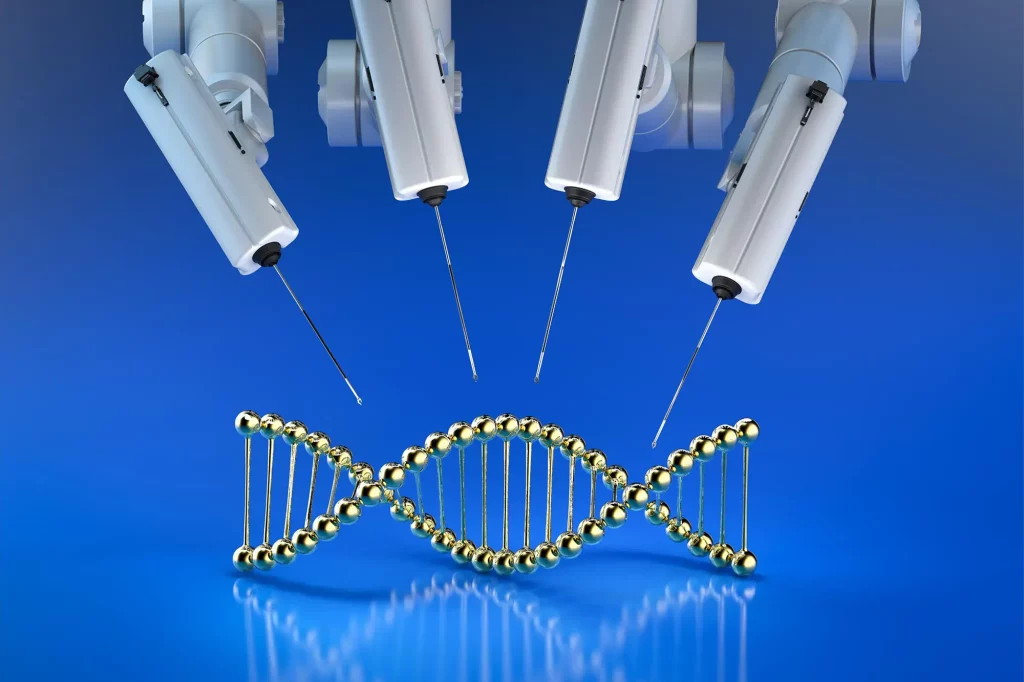
Para peneliti di University of California, Santa Barbara telah mengembangkan sebuah metode untuk secara dramatis meningkatkan efisiensi penyuntingan gen CRISPR/Cas9 tanpa menggunakan bahan virus untuk mengantarkan template gen. metode, seperti yang dijelaskan dalam makalah yang diterbitkan di Bioteknologi Alam, menggunakan ikatan silang untuk mengkatalisasi perbaikan yang diarahkan oleh homologi, sebuah langkah dalam proses penyuntingan gen, meningkatkan efisiensi tiga kali lipat tanpa meningkatkan frekuensi mutasi. Umumnya digunakan dalam kemoterapi kanker, ikatan silang ini telah ditemukan untuk meningkatkan mekanisme perbaikan alami sel dan meningkatkan kemungkinan gen yang berhasil dimodifikasi.
Para ilmuwan melipatgandakan efisiensi penyuntingan gen CRISPR/Cas9 menggunakan ikatan silang, tanpa menggunakan bahan virus untuk pengiriman. Pendekatan ini meningkatkan mekanisme perbaikan alami sel, memungkinkan pengeditan gen yang lebih tepat dan efisien, berpotensi meningkatkan penelitian penyakit dan pekerjaan praklinis.
Pengeditan gen adalah metode yang efektif untuk penelitian dan pengobatan. Sejak munculnya teknologi CRISPR/Cas9 pemenang Hadiah Nobel, alat pengeditan genom yang cepat dan tepat yang ditemukan pada tahun 2012, para ilmuwan telah bekerja untuk mengeksplorasi kemampuannya dan meningkatkan kinerjanya.
Para peneliti di University of California, Laboratorium Santa Barbara, telah menambahkan ahli biologi Chris Richardson ke kotak peralatan yang sedang berkembang ini, dengan cara meningkatkan efisiensi penyuntingan CRISPR/Cas9 tanpa menggunakan materi virus untuk mengirimkan templat genetik yang digunakan untuk menyunting urutan gen target. Menurut makalah baru mereka yang diterbitkan dalam jurnal Bioteknologi AlamMetode mereka menginduksi perbaikan yang diarahkan oleh homologi (sebuah langkah dalam proses pengeditan gen) hampir tiga kali lipat “tanpa meningkatkan frekuensi mutasi atau mengubah hasil perbaikan docking terminal.”
“Kami telah menemukan modifikasi kimiawi yang meningkatkan pengeditan gen non-virus dan kami juga menemukan jenis baru yang menarik[{” attribute=””>DNA repair,” Richardson said.
Find, Cut and Paste
The CRISPR/Cas9 method works by capitalizing on a defense technique employed by bacteria against viral attackers. To do this, the bacteria snip a piece of the invading virus’s genetic material, and incorporate it into their own in order to recognize it later. Should the bacteria get reinfected, they can target the now-familiar genetic sequences for destruction.
In gene editing, this process uses the enzyme Cas9 as molecular “scissors” to snip sequences it recognizes, guided by the CRISPR system. This cut is also an opportunity to replace the severed genes with similar (homologous) but improved ones, utilizing the cell’s natural repair mechanisms. If successful, the cell should have modified expressions and functions thereafter.
To deliver the repair template DNA to the nucleus of the cell where its genetic material lives, oftentimes viruses are used. While they are effective, the researchers say, viral workflows “are expensive, difficult to scale and potentially toxic to cells.”
Nonviral templates are potentially less expensive and more scalable, although researchers still must overcome efficiency and toxicity barriers. In their study, the Richardson Lab found that introducing interstrand crosslinks into the workflow increased homology directed repair dramatically.
“Every workflow that we have put this approach into has worked better by roughly threefold,” Richardson said.
Interstrand crosslinks are lesions that keep the double strands of a DNA helix tethered to each other, making them unable to replicate. Cancer chemotherapies use this mechanism to interrupt tumor growth and kill cancer cells. Added to a homology directed repair template, however, these crosslinks were found to stimulate the cell’s natural repair mechanisms and increase the likelihood of editing success.
“Basically, what we’ve done is taken this template DNA and damaged it,” Richardson said. “We’ve in fact damaged it in the most severe way I can think of. And the cell doesn’t say, ‘Hey this is junk; let me throw it away.’ What the cell actually says is, ‘Hey this looks great; let me stick it into my genome.’” The result is a highly efficient and minimally error-prone nonviral system of gene editing.
Their discovery, like many breakthroughs in science, was actually something of a happy accident. While working to purify proteins to study DNA repair, graduate student researcher and lead author Hannah Ghasemi noted unanticipated changes to the outcomes of their experiments.
“We were introducing these chemical modifications to the DNA templates in order to be able to pull them out of the cells and see what proteins were bound to them, and I was just checking to see if this modification had somehow affected the editing in any capacity,” she said. “I was expecting to either see no change or that it actually might have negatively affected the editing.”
What she found instead was a positive effect, up to three times the editing activity of the uncrosslinked controls. Furthermore, the team found that even with the increase in edits — and therefore the chances for errors — there was no increase in mutation frequency. They are still investigating the specific mechanisms leading to this result, but they have ideas.
“What we think happens is that the cell detects and tries to repair the damaged DNA that we’ve added this crosslink to,” Richardson said. “And in doing so, it delays the cell past a checkpoint where it would normally stop this recombination process. And so by prolonging the amount of time that it takes the cell to do this recombination, it makes it more likely that the edits will go to completion.” Studying this new process could also lead to a better understanding about how cells detect editing reagents and how they “decide” to accept them or not, he said.
This method will find the most use in ex-vivo gene editing applications, according to the team, that is, in the realm of disease research and preclinical work.
“We can more effectively knock down genes and insert things into genomes to study systems outside of the human body in a lab setting,” Ghasemi said. This development allows them to more efficiently build disease models and test hypotheses about how diseases work, which could lead to better clinical and therapeutic approaches.
Reference: “Interstrand crosslinking of homologous repair template DNA enhances gene editing in human cells” by Hannah I. Ghasemi, Julien Bacal, Amanda C. Yoon, Katherine U. Tavasoli, Carmen Cruz, Jonathan T. Vu, Brooke M. Gardner and Chris D. Richardson, 27 February 2023, Nature Biotechnology.
DOI: 10.1038/s41587-022-01654-y

“Kutu buku musik lepas. Pecandu internet bersertifikat. Pencinta perjalanan. Penyelenggara hardcore. “










More Stories
Makanan cepat saji dan daging olahan mungkin menjadi penyebab kanker di kalangan anak muda: dokter
Kapan peluncurannya? Bisakah saya melihatnya di New Smirna?
Sebuah studi baru memetakan awal mula kehidupan hewan